MUcluster
High-density surface electromyography (HDEMG) provides a noninvasive insight into the electrical activity of skeletal muscles. It can be used to monitor muscle activation and the participation of different muscles in different movements and to infer the state and properties of the neuromuscular system in humans. In the last two decades, methodologies have been developed for the computational decomposition of surface EMG signals into contributions from individual motor units (MUs) and, thus, the identification of neural codes with which the brain controls skeletal muscles. Despite the remarkable progress and numerous new neurophysiological insights enabled by this methodology, the identification of MUs still has the following limitations:
- When compared to the several hundreds of MUs active in individual muscles, the number of MUs identified from HDEMG is relatively low. During the decomposition process, only accurately identified MUs are kept for further analysis, whereas many MUs that do not meet strict quality measures are discarded. This leads to the relatively low percentage of the energy of HDEMG signals accounted for by decomposition and challenges the representativeness of the identified group of MUs.
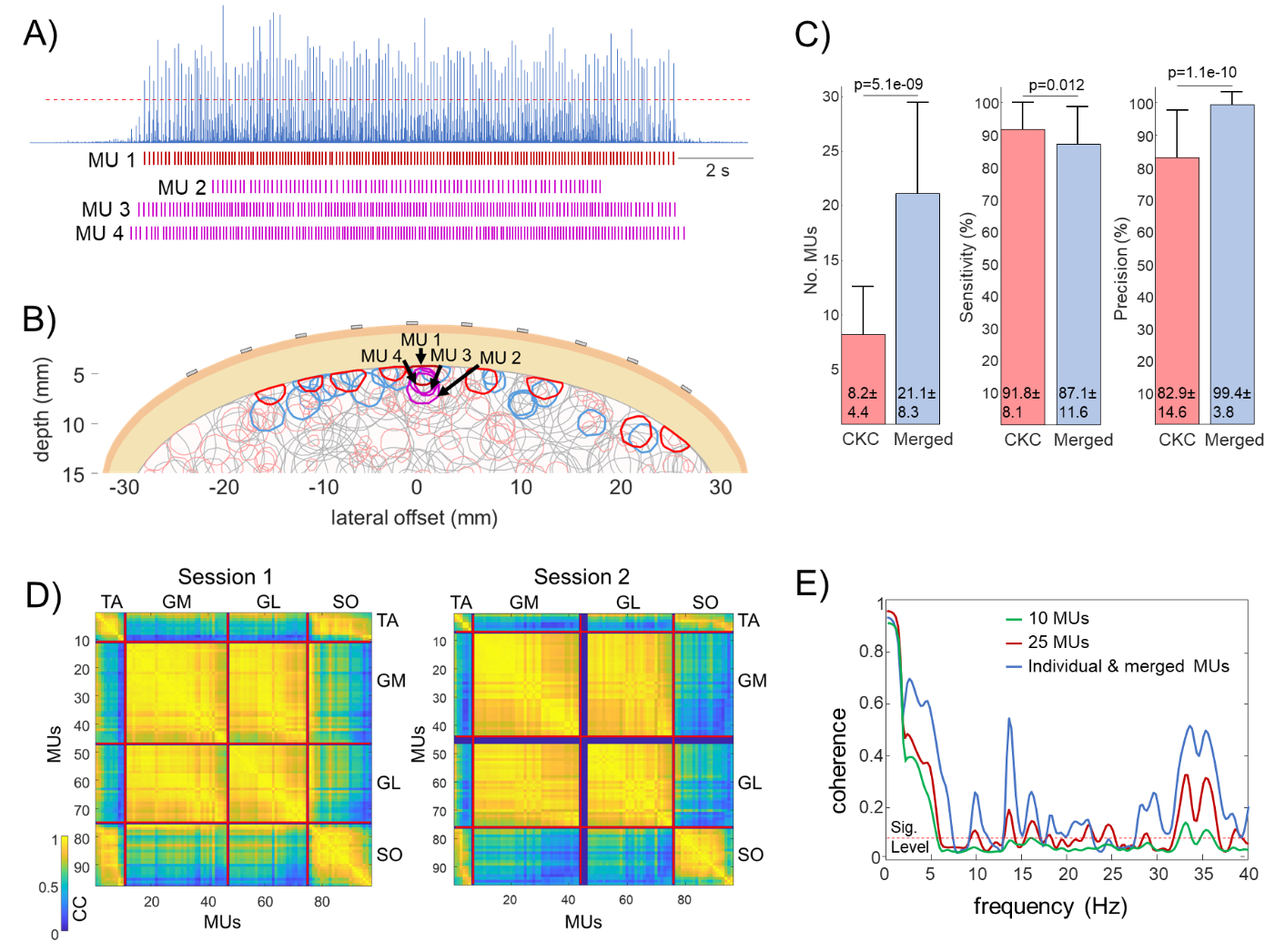
- When identifying MUs from HDEMG signals, we often encounter merged MU pulse trains that cannot be separated using mathematical procedures and are, therefore, usually discarded in the analysis process. This also discards a significant amount of useful information.
- Merged spike trains reflect the common anatomic properties of identified MUs in HDEMG. However, it is not clear to what extent these MUs also belong to the same functional clusters.
- Most studies on the excitation of skeletal muscles have been performed in isometric contractions, and it is unknown to what extent are the MU clusters shared among the isometric and dynamic contractions and how they change over time.
- Nonlinear motoneuron transfer functions hinder the effectiveness of currently proposed linear MU clustering techniques. Nonlinear techniques have been proposed outside the MU identification community but, to the best of our knowledge, have not been systematically tested on MU populations.
- There is a significant sex bias in the results of MU identification. The MU identification in females has been demonstrated to be more challenging, and this discrepancy between the sexes has not yet been sufficiently addressed. The extent of MU merging in females has not been systematically studied and compared to males.
- Several frequently investigated skeletal muscles yield a low number of identified MUs. Indeed, most of the studies of MU behaviour from surface EMG have been conducted on the distal muscles that are relatively easy to decompose, whereas the other groups of muscles have not been sufficiently investigated by decomposition methodology.
- Current decomposition procedures are semi-automatic and extensive post-processing by manual or computer-aided editing is still required. This consumes a lot of energy and time of a human operator and puts the objectivity of decomposition under question. Both factors severely limit the exploitation of the decomposition results and the transfer of decomposition techniques into mature technology.
In this project, we propose to investigate and answer the following research questions:
- What are the key factors contributing to MU merging in different skeletal muscles and across different conditions (isometric vs. dynamic vs. elicited contractions), different muscles (distal vs. proximal) and different sexes (female vs. male)?
- By how much can we increase the number of identified MUs if we take merged MUs into account, also in muscles with traditionally low MU yield?
- How does the MU merging (e.g. anatomical clustering in HDEMG decomposition) relate to the functional coupling of MUs?
- What nonlinear techniques can be used for functional clustering of MU discharge patterns, including the merged MUs, and how effective are they in different experimental conditions?
- How does the functional clustering of MUs change across different conditions (isometric vs dynamic, voluntary vs elicited contractions), different muscles (distal vs proximal) and different sexes (female vs male)?
- To what extent can the analysis of merged MUs reduce the need for time-consuming manual editing of HDEMG decomposition results and increase the robustness of HDEMG decomposition to noise, the thickness of adipose tissue and different experimental conditions?
The project will be conducted by researchers at the Faculty of Electrical Engineering and Computer Science (UM FEECS) of the University of Maribor, Slovenia and the Science and Research Centre Koper (SRC Koper), Slovenia. We will collaborate with external partners from Loughborough University (LU), Leicestershire, UK, and Imperial College London (ICL), UK.